In mice, researchers have discovered the presence of ‘oncostreams,’ highly active cells connected to how brain tumors grow and invade healthy tissue.
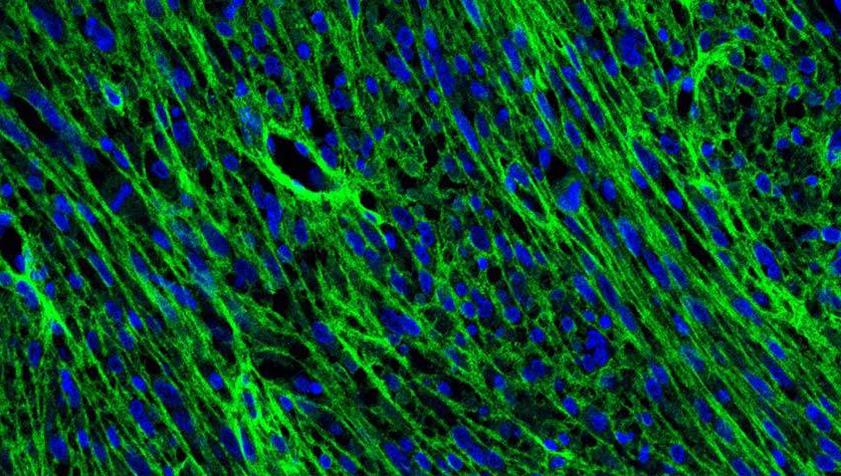
Tumors are made up of many types of cells, both cancerous and benign. The specific complexity of the cells inside brain tumors has been a trademark of the disease, one that makes treatment extremely difficult. While scientists have long known about the variety of cells within a brain tumor, the ways these tumors grow have relied on the understanding that the cells are static, unmoving, and relatively fixed.
But researchers at the University of Michigan Department of Neurosurgery and Rogel Cancer Center have discovered that these aggressive tumors contain highly active cells that move throughout tissue in complicated patterns. What’s more, the accumulations of these elongated, spindle-like cells found throughout the tumor, coined ‘oncostreams,’ serve as the basis for cancerous cells’ behavior, determining how tumors grow and invade normal tissue.
Pedro Lowenstein, M.D., Ph.D., Richard C. Schneider Collegiate Professor of Neurosurgery and lead author of the study in Nature Communications, says this organized growth is what makes brain tumors so relentless.
“Brain tumors are highly lethal, with less than 5% of patients living beyond five years,” he said. “Unfortunately, reoccurrence is what eventually kills patients. They receive surgery for their initial tumor, but the tumor always comes back within 12 to 18 months,” he said.
Lowenstein and his team, including Maria Castro, Ph.D., also found that overexpression of Collagen 1, a protein produced by tumor cells, is essential to the growth and function of these structures.
“When we eliminated Collagen 1 production from tumor cells, the animal models with brain tumors lived much longer. This step removes oncostreams from tumors and reduces tumor aggressive behavior because the tumors need Collagen 1 to move in the specific way we discovered,” said Lowenstein.
Lowenstein says this structure is likely present in other types of cancer too. “Once people recognize that there are dynamic areas of the tumor and that they’re related to tumor growth, eventual invasion, and death, people will likely locate oncostreams in other tumor models,” he said.
To detect this previously unknown presence of oncostreams, the team collaborated with Todd Hollon, M.D., assistant professor in the Michigan Medicine Department of Neurological Surgery, and Sebastien Motsch, Ph.D., associate professor of mathematics at Arizona State University, to implement artificial intelligence methods to identify the structures in tissue.
“Essentially, we showed images to a computer and the computer eventually learns to recognize oncostreams,” Lowenstein explained.
Dismantling oncostreams through the removal of Collagen 1 could represent a novel therapeutic target to treat lethal brain tumors.
“This research proves the crucial importance of continuing to investigate the complicated extracellular matrix,” notes Andrea Comba, Ph.D., research investigator and first author of the study.
“Based on this discovery, we propose targeting tumor collagen to disrupt oncostreams, and as novel therapy for the treatment of brain glioma,” she said.
Funding: National Institutes of Health, National Institute of Neurological Disorders and Stroke grants (R37-NS094804, R01-NS105556, R21-NS107894, R21-NS091555, R01-NS074387); National Institute of Neurological Disorders and Stroke grants (R01-NS076991, R01-NS096756, R01-NS082311, R01-NS122234, R01-NS127378); National Institute of Biomedical Imaging and Bioengineering (R01-EB022563); National Cancer Institute (U01CA224160); Rogel Cancer Center at The University of Michigan (G023089); Ian’s Friends Foundation grant (G024230); Leah’s Happy Hearts Foundation grant (G013908); Pediatric Brain Tumor Foundation grant (G023387); ChadTough Foundation grant (G023419); RNA Biomedicine grant (F046166); National Cancer Institute grants: (R01 CA125577, R01 CA107469); Health and Human Services; National Institutes of Health ( UL1 TR002240); Michigan Institute for Clinical and Health Research; Postdoctoral Translational Scholars Program (Project F049768.)
Paper cited: “Spatiotemporal analysis of glioma heterogeneity reveals COL1A1 as an actionable target to disrupt tumor progression,” Nature Communications. DOI: 10.1038/s41467-022-31340-1
This story was originally published by the Michigan Health Lab Blog on June 28, 2022.