Discovering a puddle of mouse urine seems like a strange scientific “eureka” moment. But for one research team, it’s exactly what led to a new discovery.

Discovering a puddle of mouse urine seems like a strange scientific “eureka” moment. But for one research team, it’s exactly what led to a new discovery. The researchers’ findings may enhance understanding of how our bodies balance water content — 50 to 60 percent of our weight. It may also lead to better understanding of hormone-related diseases that can cause conditions ranging from diabetes to obesity.
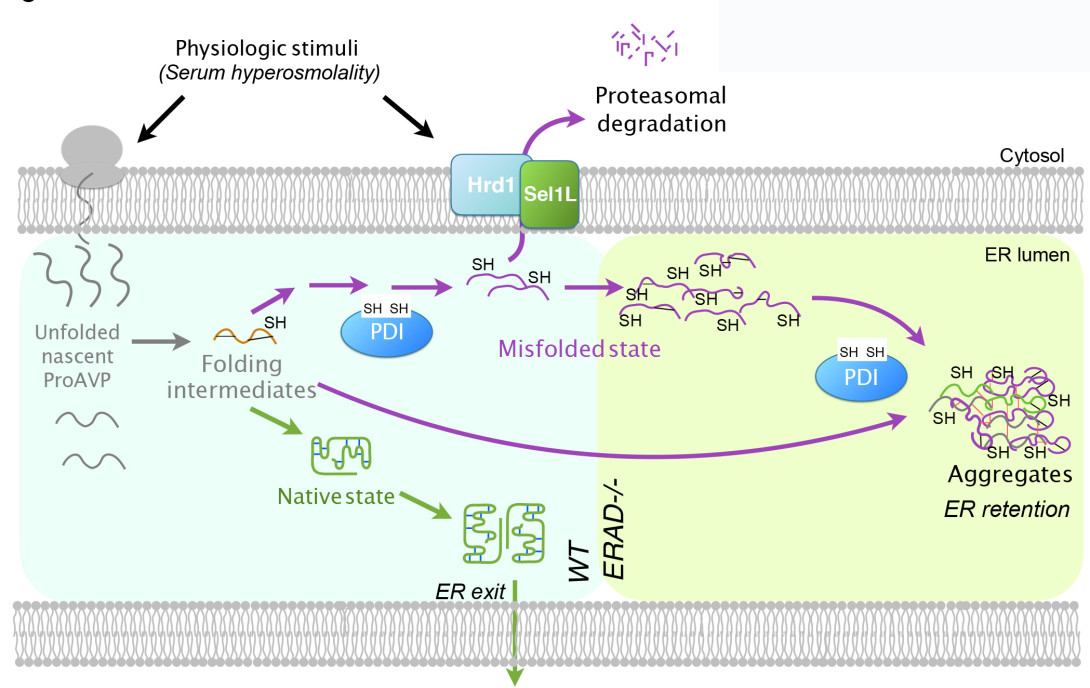
Starting from that abnormally wet cage litter, the researchers traced the cause all the way into tiny nerve cells in the mice’s pituitary glands. Clues and experiments led them into the folds of the endoplasmic reticulum, or ER. The ER is the last stop for many items that cells send out into the world. If the pituitary gland cell is a hormone factory, then the ER is both the quality control department and the packaging and shipping department for those hormones.
The team’s discovery shows that the ER quality control machine does more than just keep the cell from shipping poorly made goods, destroying bad products before they do damage. In addition, the ER machinery provides a safe space for the cell to put finishing touches on its products, keeping the bad ones from clumping together with the good ones, the research finds. If this safe space fails, then the hormone vasopressin can’t do a key job: helping the body reclaim water from urine and balance its water levels.
What might one see in a mouse that lets too much water go in its urine? Perhaps an unusually wet mouse cage. University of Michigan physiology professor Ling Qi, Ph.D., explains that the findings, published in the Journal of Clinical Investigation, illustrate a new role for a process called endoplasmic reticulum-associated degradation, or ERAD.
“We know that critical events regulating hormone levels in the body happen in the ER,” he says. “We demonstrate that the machinery of the ERAD process controls the level of hormones released from cells and thereby controlling systemic water balance.”
A New Role for ERAD
ERAD was already known to play a key role in identifying badly made products, such as misfolded proteins, and breaking them down before they can leave the cell. In fact, many genetic diseases may result from overwhelming the ERAD machinery with too many mutated, misfolded proteins. Even during normal protein production, a few bad copies get made and fold inappropriately. U-M has started a Protein Folding Diseases Initiative to study these issues.
Qi, along with postdoctoral fellows Guojun Shi, Ph.D., and Geun Hyang Kim, Ph.D., and undergraduate student Diane Somlo, now a Yale University medical student, and colleagues from around the world, show that ERAD can do more. Their study centers on proAVP, a “prohormone” precursor of the hormone arginine-vasopressin, also sometimes called vasopressin or antidiuretic hormone.
They had actually set out to study what role another molecule, called Sel1L, plays in living organisms. They did not expect that Sel1L was important to water balance and the ability of proAVP to leave cells. So, they produced a mouse equipped with a drug-sensitive genetic mechanism that allowed them to turn off Sel1L production. When they stopped Sel1L production, the mice started drinking a lot more water and producing watery urine, mirroring symptoms seen in people with a rare hormone disorder known as diabetes insipidus.
The disease, which occurs in about 1 in 25,000 people, is known to be caused by a lack of vasopressin, or decreased ability of kidneys to respond to it. Through painstaking research, the team showed that when ERAD didn’t happen normally, misfolded proAVP molecules were not cleared, and they formed aggregates, or clumps, with normal proAVP molecules. That meant the normal hormone couldn’t get where it needed to go.
“What’s really fascinating is that ERAD appears to control secretion of AVP by degrading misfolded proteins and allowing the good ones to go,” says Qi. “Without ERAD, the bad ones attach to the good ones, which then stay in the ER. This suggests that central diabetes insipidus may develop because of problems with quality control in the ER.” The researchers suspect that the water level in the body may even act as a feedback loop to trigger more ERAD production. But if the ERAD machinery is broken, that extra prohormone could gum up the works further.
Taken together, the findings suggest that efforts to boost ERAD function could help normal prohormone folding and shipping go more smoothly. Perhaps targeted drugs could be found to accomplish this, says Qi, though that could be years away.
“Protein folding is critical in the pathogenesis of many diseases,” he says. “This shows how important the ERAD process is in that process. If ER stress response is a fire alarm, ERAD is the firefighters putting out the fires and preventing them from spreading to the next house.”
In addition to Qi, Shi and Kim, who are members of the Department of Molecular and Integrative Physiology at the U-M Medical School, and their U-M colleague Peter Arvan of the Department of Internal Medicine, the research team included Cristina Prescianotto-Baschong, Nicole Beuret, Jonas Rutishauser and Martin Spiess of the University of Basel, Switzerland, Shengyi Sun of the University of Texas Southwestern Medical Center, and Qiaoming Long of Suzhou University in China.
The research used the Microscopy & Image Analysis Laboratory at the U-M Medical School, and funding from the U-M Protein Folding Diseases Initiative as well as the National Institutes of Digestive and Diabetes and Kidney Diseases (DK48280, DK111174), the Swiss National Science Foundation and the American Diabetes Association.
This story was originally published by the Michigan Health Lab Blog on September 19, 2017.