New V2 IACUC Protocol Application Form
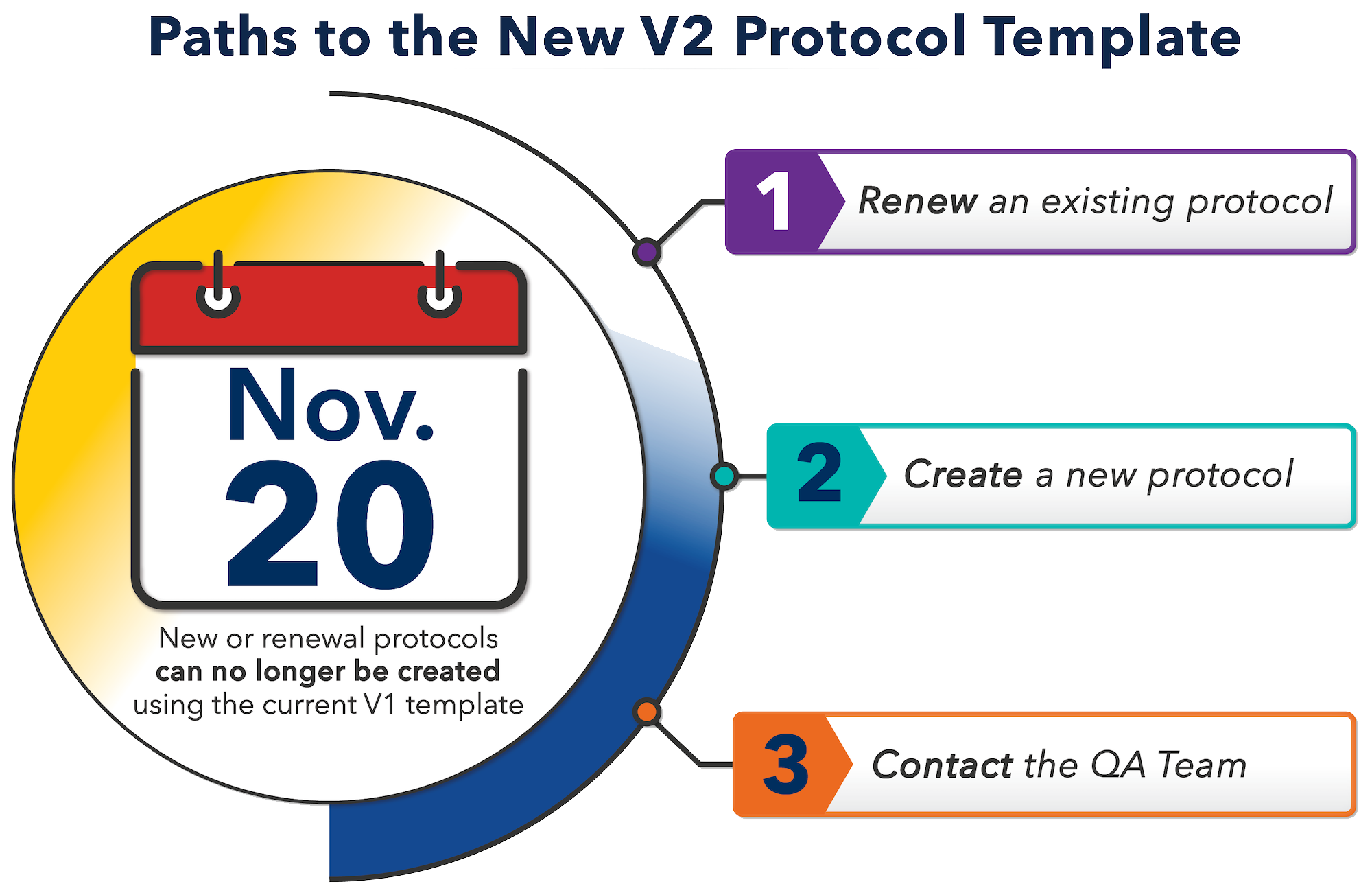
Effective November 20, 2023, the eResearch Animal Management (eRAM) system used for managing the submission, review, and approval of all applications/protocols involving the use of vertebrate animals at the U-M has launched a new protocol template – commonly referred to as “V2.”
After this date, Study Teams will no longer be able to create new or renewal protocols using the current (“V1”) template. All V1 protocols that have already been initiated prior to this date will continue through the IACUC review and approval process, and no duplicate information will need to be entered.
Transitioning to the New V2 Template
As existing protocols begin to expire over the next three years, all protocols using the current V1 template will be transitioned to the new V2 format.
Given the many layout and structural changes (e.g., minimal number of open entry text boxes, addition of picklists and checkboxes, default text and pre-populated fields for common procedures) between the two templates, information from an existing V1 protocol will not automatically copy to the new V2 template at the time of protocol renewal.
Key Changes
The Animal Care & Use Office (ACUO) has spent several years diligently evaluating and fine-tuning the V2 protocol template, with the primary goal of minimizing the time and effort required of researchers to complete the form.
Examples of key changes that researchers can expect include:
- The number of open entry text boxes has been minimized
- Much of the form will now be populated using picklists and checkboxes, which will eliminate the need to enter duplicative, and possibly conflicting, information across different protocol sections
- Default text and pre-populated fields will include programmatic recommendations for common procedures (e.g., aseptic procedure)
- Increased flexibility to request research-specific plans and dosages (e.g., requesting a “maximum” number of injections instead of having to select specific values)
Getting Started with a V2 Protocol
After November 20, 2023:
- The V2 template will be used for all new and renewing protocols. Researchers with an expiring protocol will be contacted by a member of the Quality Assurance (QA) Team approximately 90 days before protocol expiration to help facilitate a smooth transition to the new template.
- Any PI who is new to eRAM and/or creating an animal use protocol at the U-M for the first time will also be contacted by the QA Team and offered assistance with completing the V2 template.
QA Team assistance can be requested by emailing [email protected] or by accessing the QA Team’s Virtual Office Hours Queue via Zoom. Office Hours are available on a drop-in basis Monday – Friday, 9:00 AM – 4:00 PM.
V2 FAQs
V1 protocols will be transitioned to the new V2 protocol template at the time of protocol expiration.
A PI or PI Proxy (as defined by eRAM) may also contact the Quality Assurance Team in the Animal Care & Use Office ([email protected]) to initiate a new V2 protocol at any time.
The amount of time it takes to transition to the new V2 template will vary greatly depending on the complexities of each individual protocol.
Given the many layout and structural changes (e.g., minimal number of open entry text boxes, addition of picklists and checkboxes, default text and pre-populated fields for common procedures) between the two templates, protocol renewal may take longer as information from an existing V1 protocol will not automatically copy to the new template.
Study Teams are strongly encouraged to work with a member of the QA Team to facilitate a smooth transition of any existing V1 protocol.
After November 20, 2023, Study Teams will no longer be able to create a new or renewal V1 protocol in eRAM. As an existing V1 protocol approaches expiration, Study Teams will be prompted to renew their protocol using the new V2 template.
Any V1 protocol that is not renewed/transitioned to the new V2 template will expire and no longer be approved for animal use.
Users with an approved V1 protocol will continue to make amendments in the V1 template until the protocol expires or is canceled.
- The number of open entry text boxes has been minimized
- Much of the form will now be populated using picklists and checkboxes, which will eliminate the need to enter duplicative, and possibly conflicting, information across different protocol sections
- Default text and pre-populated fields will include programmatic recommendations for common procedures (e.g., aseptic procedure)
- Increased flexibility to request research-specific plans and dosages (e.g., requesting a “maximum” number of injections instead of having to select specific values)
This change was made after extensive feedback from the U-M research community and in concert with updates to the regulatory standards that govern the use of animals in research and instruction.
The V2 template was specifically created to:
- Minimize the time and effort required of researchers to complete a protocol, thus reducing administrative burden, and
- Standardize common practices and procedures to ensure animal welfare.
No, transitioning to the new V2 template will NOT impact how the eRAM system communicates with other U-M platforms used to manage animal use/grant projects.
To date, it is estimated that more than 100 protocols are using the new V2 template.
References and Resources
QA Team Virtual Office Hours
Valid U-M login credentials required to access
Questions?
If you have questions about the new V2 protocol template or would like assistance with transitioning your existing V1 protocols, please contact the Quality Assurance Team in the Animal Care & Use Office at [email protected].