Controlled Substances Drugs Guidelines IACUC Policies
New Resources to Promote Responsible Use of Drugs and Other Medical Materials
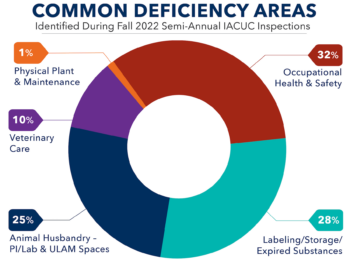
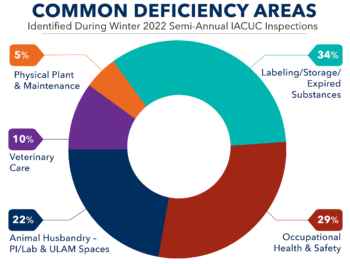
The appropriate use of drugs and other medical materials – fluids, vehicles, biologics, and drugs – is critical for protecting animal welfare, ensuring the validity of your results, and avoiding incidents of non-compliance. Given the seriousness of these concerns, recent updates to several programmatic documents, and an analysis of areas identified for continued refinement through ...